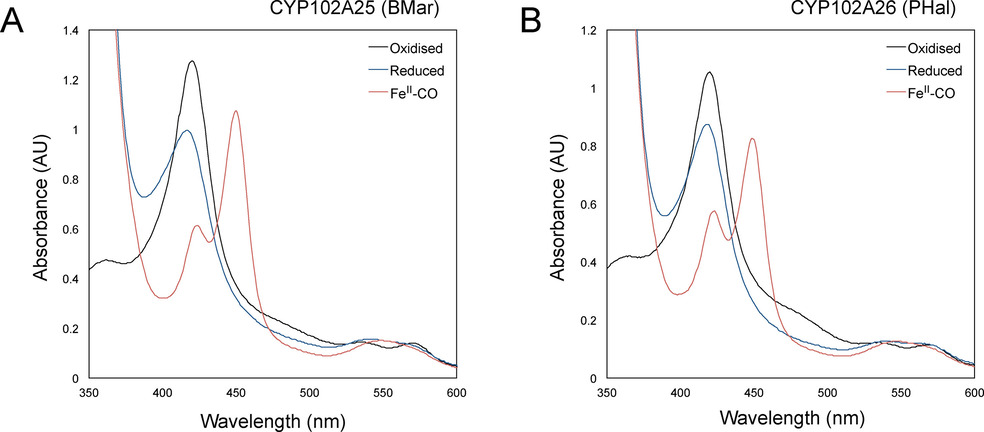
Cytochrome P450 monooxygenases are highly desired biocatalysts owing to their ability to catalyse a wide variety of chemically challenging C−H activation reactions. The CYP102A subfamily of enzymes are natural catalytically self‐sufficient proteins consisting of a haem and FMN‐FAD reductase domain fused in a single‐component system. They catalyse the oxygenation of saturated and unsaturated fatty acids to produce primarily ω−1, ω−2 and ω−3 hydroxy acids. These monooxygenases have potential applications in biotechnology; however, their substrate range is still limited and there is a continued need to add diversity to this class of biocatalysts. Herein, we present the characterisation of two new members of this class of enzymes, CYP102A25 (BMar) from Bacillus marmarensis and CYP102A26 (PHal) from Pontibacillus halophilus, both of which express readily in a recombinant bacterial host. BMar exhibits the highest activity toward myristic acid and shows moderate activity towards unsaturated fatty acids. PHal exhibits broader activity towards mid‐chain‐saturated (C14–C18) and unsaturated fatty acids. Furthermore, PHal shows good regioselectivity for the hydroxylation of myristic acid, targeting the ω−2 position for C−H activation.