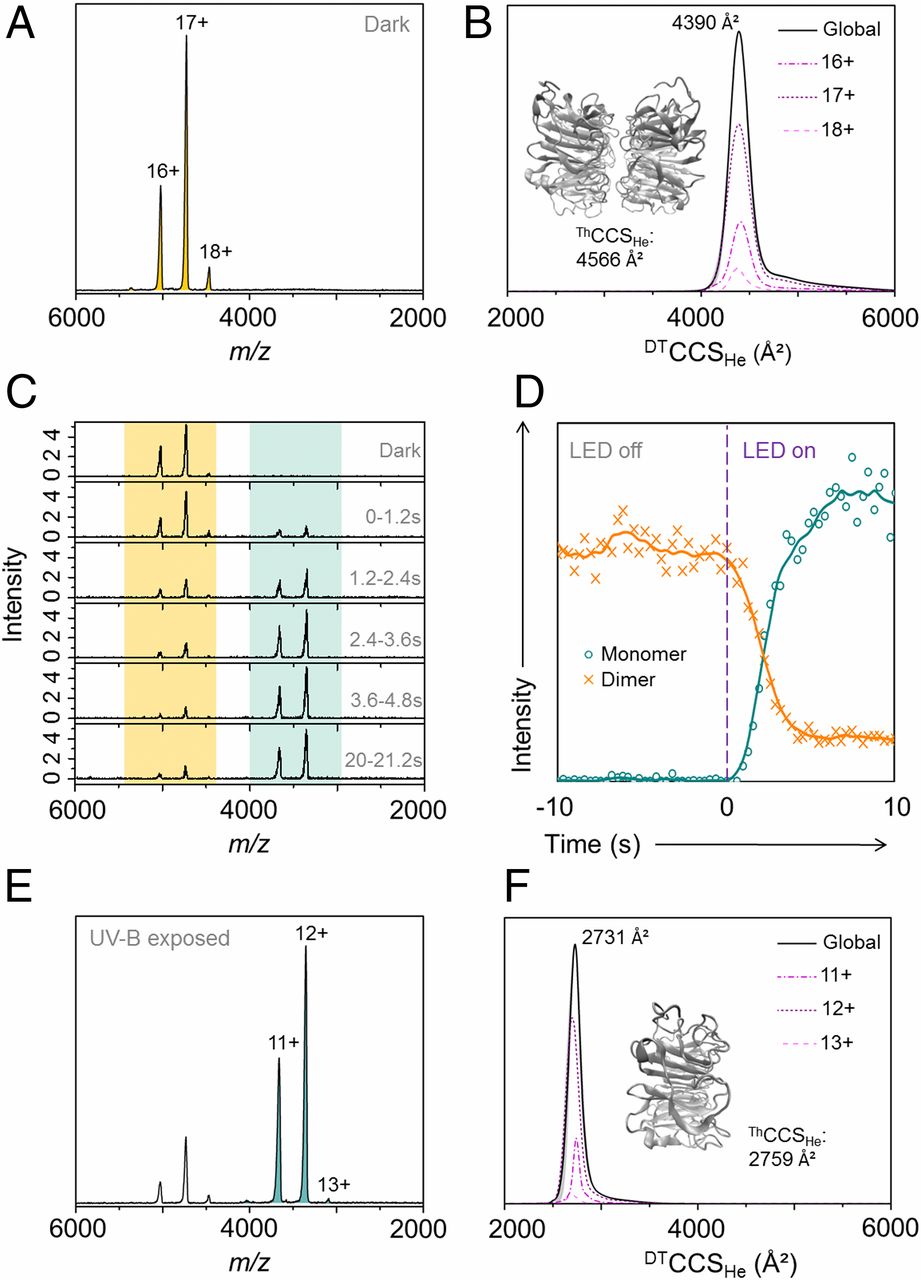
UVR8 is a plant photoreceptor protein that regulates photomorphogenic and protective responses to UV light. The inactive, homodimeric state absorbs UV-B light, resulting in dissociation into monomers, which are considered to be the active state and comprise a β-propeller core domain and intrinsically disordered N- and C-terminal tails. The C terminus is required for functional binding to signaling partner COP1. To date, however, structural studies have only been conducted with the core domain where the terminal tails have been truncated. Here, we report structural investigations of full-length UVR8 using native ion mobility mass spectrometry adapted for photoactivation. We show that, while truncated UVR8 photoconverts from a single conformation of dimers to a single monomer conformation, the full-length protein exists in numerous conformational families. The full-length dimer adopts both a compact state and an extended state where the C terminus is primed for activation. In the monomer the extended C terminus destabilizes the core domain to produce highly extended yet stable conformations, which we propose are the fully active states that bind COP1. Our results reveal the conformational diversity of full-length UVR8. We also demonstrate the potential power of native mass spectrometry to probe functionally important structural dynamics of photoreceptor proteins throughout nature.
Inês S. Camacho a,b,c,d,1, Alina Theisen a,b,c,1, Linus O. Johannissen a,c, L. Aranzazú Díaz-Ramose
, John M. Christie e, Gareth I. Jenkins e, Bruno Bellina a,b,c,2, Perdita Barran a,b,c,2, and Alex R. Jones a,b,c,d,2
a School of Chemistry, The University of Manchester, Manchester M13 9PL, United Kingdom; b
Photon Science Institute, The University of Manchester, Manchester M13 9PL, United Kingdom; c
Manchester Institute of Biotechnology, The University of Manchester, Manchester M1 7DN, United Kingdom; d Biometrology, Division of Chemical Medical and Environmental Sciences, National Physical Laboratory, Teddington TW11 0LW, United Kingdom; and e Institute of Molecular, Cell and Systems Biology, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, United Kingdom