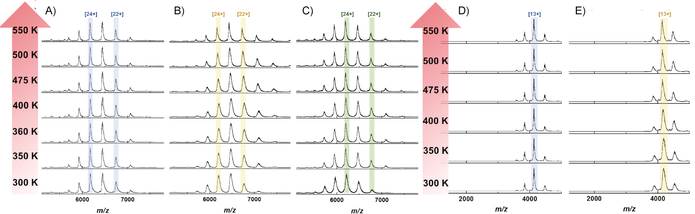
The aggregation of protein-based therapeutics such as monoclonal antibodies (mAbs) can affect the efficacy of the treatment and can even induce effects that are adverse to the patient. Protein engineering is used to shift the mAb away from an aggregation-prone state by increasing the thermodynamic stability of the native fold, which might in turn alter conformational flexibility. We have probed the thermal stability of three types of intact IgG molecules and two Fc-hinge fragments by using variable-temperature ion-mobility mass spectrometry (VT-IM-MS). We observed changes in the conformations of isolated proteins as a function of temperature (300-550 K). The observed differences in thermal stability between IgG subclasses can be rationalized in terms of changes to higher-order structural organization mitigated by the hinge region. VT-IM-MS provides insights into mAbs structural thermodynamics and is presented as a promising tool for thermal-stability studies for proteins of therapeutic interest.
Kamila J. Pacholarz, Shirley J. Peters, Rachel A. Garlish, Alistair J. Henry, Richard J. Taylor, David P. Humphreys and Perdita E. Barran
ChemBioChem, 2016, 17, 46-51.